what recommendation is made for pregnant women to ensure they consume adequate iodine?
- Research article
- Open Access
- Published:
Iodine nutritional status of women in their outset trimester of pregnancy in Catalonia
BMC Pregnancy and Childbirth volume 17, Article number:249 (2017) Cite this article
Abstract
Background
Sufficient iodine intake is needed during pregnancy to ensure proper fetal evolution. The iodine levels of women in their beginning trimester of pregnancy in Catalonia are currently unknown. This data would help to determine whether our public wellness services should found recommendations or interventions in this line. The aim of this study was to investigate the iodine nutritional status, prevalence of urinary iodine <150 μg/L, and tobacco use in the offset trimester of pregnancy in our setting.
Methods
Cross-sectional study. Data were collected during 2008–2009 from women in their first trimester at the primary care centers of the province of Barcelona (Spain). Pregnant women included in the study completed a questionnaire on eating habits and underwent urinary iodine concentration (UIC) assessment.
Results
Nine hundred xl five women completed the dietary questionnaire and urinary iodine testing. Median UIC was 172 μg/L, with 407 participants (43.1%) showing levels <150 μg/L. On multivariate logistic regression analysis, intake of 1–ii glasses of milk per mean solar day, OR = 0.636 95% CI (0.45–0.ninety) or >2 glasses, OR = 0.593 95% CI (0.37–0.95); iodized salt consumption, OR = 0.678 95% CI (0.51–0. 90); and use of iodine supplementation, OR = 0.410 95% CI (0.31–0.54), protected against the adventure of UIC <150 μg/L. Simultaneous consumption of iodized table salt and milk (≥i glass/day) showed a larger protective effect: OR = 0.427, 95% CI (0.31–0.54).
Determination
The median UIC of the significant women surveyed indicated an acceptable iodine nutritional condition according to the criteria established by the WHO and ICCIDD. The take a chance of urinary iodine <150 μg/50 decreased with simultaneous consumption of milk and iodized common salt, similar to the decrease seen with iodine supplementation.
Background
A recent nationwide report conducted in Espana has reported that iodine nutrition is acceptable in the general population, which showed a median urinary iodine concentration (UIC) of 117 μg/L [1]. Nevertheless, in the subgroup of women of childbearing historic period, median UIC was 114 μg/L, a value indicating a situation of chance in pregnancy [i].
Iodine is an essential micronutrient that must be supplied by regular intake in the nutrient consumed. It has a vital function in the synthesis of thyroid hormones, which human activity on several organs and body systems, and is especially of import for central nervous system development starting from the earliest stages of embryonic and fetal growth [two].
Iodine requirements vary with age and the private's physiological condition. In pregnancy, thyroid metabolism undergoes a serial of changes equally the demand for iodine increases due to embryonic and fetal development. Since the early stages of pregnancy, the thyroid gland undergoes stimulation due to the effect of chorionic gonadotropin hormone [3]. In that location is an increment in the available claret volume and the iodine distribution infinite, iodine is transferred from the mother's circulatory system to the fetal-placental unit, and an increment in glomerular filtration leads to increasingly larger amounts of iodine being eliminated in urine (30%–50%). This combination of factors results in a high demand for iodine during pregnancy [four,5,6]. During the first trimester, thyroid hormones for embryonic and fetal tissues rely exclusively on the available maternal hormones; hence, iodine deficiency in the mother may have a negative impact on prenatal development [7]. Clinical studies accept reported a relationship of iodine deficiency and maternal hypothyroxinemia during pregnancy with dumb neurocognitive and psychological development of the children [3, eight,9,ten,eleven,12,thirteen]. Therefore, it is important to increase iodine intake starting in the early stages of pregnancy and, if possible, even beforehand. The World Health Organization (WHO), together with the United nations International Children's Emergency Fund (UNICEF) and the International Council for Command of Iodine Deficiency Disorders (ICCIDD), recommend a daily iodine intake of between 200 and 250 μg/day during pregnancy [14].
Under normal conditions, in that location is a balance between iodine intake and urinary output, making the UIC a skilful indicator of recently consumed iodine [15]. The median UIC indicating optimum iodine intake in meaning women is 150 to 250 μg/Fifty [fourteen, 15]. Studies carried out in diverse European countries, including Spain, accept reported highly variable values, ranging from sixty to 250 μg/L [16].
Several strategies can be applied to ensure an adequate iodine supply and to correct deficiencies in this regard. The WHO and other international agencies have advocated universal common salt iodization for worldwide consumption. In cases where iodine requirements cannot be guaranteed through diet during pregnancy, the WHO recommends potassium iodide supplementation [14].
Another attribute to include when considering the dietary and health habits of pregnant women is tobacco use and its bear on on maternal thyroid function. Tobacco is considered a goitrogenic substance, every bit it inhibits thyroid uptake of iodine [17]. Tobacco use during pregnancy has been associated with changes in thyroid function in both the mother and fetus [18,19,xx], and with changes in the UIC [21]. During lactation, tobacco use lowers the iodine content in breast milk [22].
Currently, at that place is footling available data on the iodine nutritional status in significant adult female in Catalonia (an autonomous region in northeast Spain) or the dietary habits that may exist determinant in this regard. The available data comes from studies conducted in limited areas of our region or in women in the third term of pregnancy [23, 24]. To make full this gap, the aim of this study was to determine the iodine status of a population of pregnant women during the first trimester of pregnancy—the time when thyroid deficiency can have the greatest impact on embryonic development—and to investigate the habits affecting the iodine status in this population in a large geographical area of Catalonia. The ultimate aim was to gain information that will be useful to assess the need for interventions in this population by a public wellness program.
Methods
Blueprint
This is a cross-sectional descriptive study, based on information nerveless from women in their outset trimester of pregnancy in 2008–2009. The information was obtained within the construction of a clinical trial whose ultimate purpose is to evaluate the outcome of an intervention on eating habits and its benefits on iodine levels in pregnant women [25]. The present written report describes the iodine condition results in this population. The study was approved by the Clinical Inquiry Ideals Commission at the Principal Care Research Institute (IDIAP) Jordi Gol.
Setting
The study was conducted within the framework of the primary intendance middle Program for Sexual and Reproductive Health Care (PASSIR) of the Catalonia Central and Metropolitana Nord Regional Offices of the Institut Català de la Salut (ICS, Catalonian Plant of Health) in the province of Barcelona (Spain).
Participants
A sequent recruitment was carried out within a clinical trial framework. During 2008 and 2009, all women older than 17 years who were seen in the participating centers in their start trimester of pregnancy (<13 weeks) and accepted to participate were included in the study. Pregnant women with thyroid disease, no telephone contact, or difficulty communicating with the health personnel (cognitive, sensory, or language problems), and those refusing to participate, were excluded. A calculated sample size of 989 pregnant women was needed to reply the hypothesis of the clinical trial [25].
Data collection
The socio-demographic data included patient historic period, place of origin, place of residence (rural/urban), and educational level. Information on dietary and other habits was collected at a personal interview by midwives in the participating primary care centers, using a standardized questionnaire [25] showing skilful reliability (Cronbach'due south alpha, 0.960 and intraclass correlation coefficient, 0.927). The questionnaire independent items related to consumption of moo-cow milk (glasses/day; 1 glass = 200 mL), and fish (servings/week), regular consumption of iodized salt (Yes/No), daily use of iodine supplements (potassium iodide or iodine vitamin supplements) (Yes/No), use of iodinated antiseptics, and tobacco use.
Urinary iodine concentration (μg/L) was determined as follows: A first morn urine sample was collected from each adult female, rapidly frozen at −forty °C, and transported within 24 to 48 h to a central laboratory (Barcelona Infirmary Clinic), where UIC decision was performed using the Benotti & Benotti method [26]. Urine was outset digested with chloric acrid and then underwent the Sandell-Kolthoff reaction, in which iodine was determined past its action equally a catalyst in the reduction of ceric ammonium sulphate in the presence of arsenious acid. The inter-assay and intra-analysis coefficients of variation of the technique were 15.5% and 12.vi%, respectively. Three times a year, the UIC analysis undergoes evaluation by an external quality assessment program from the Spanish Association of Neonatal Screening (AECNE). UIC values were dichotomized as <150 μg/50 (insufficient) and ≥150 μg/Fifty (adequate) [fourteen] in the analyses.
Statistical assay
Quantitative variables are described as the mean and standard difference or the median and get-go and tertiary quartiles (Q1-Q3) for those with a non-normal distribution. Categorical variables are expressed every bit the accented frequency and percentage.
The Pupil t test for independent data and the Mann-Whitney U exam, as appropriate, were used for quantitative variables and the Pearson chi-square examination for categorical variables.
A multivariate logistic regression model was performed, in which the dependent variable was UIC less than or equal to 150 μg/L. The Initial model contained all the covariates that individually had an association with UIC co-ordinate to a significance level of p ≤ 0.ane. The concluding model included statistically pregnant (p < 0.05) covariates, while also because Akaike's information criterion and biological plausibility. Akaike'due south information criterion is a tool that balances the data provided past a set of variables (goodness of fit) and the principle of parsimony (use of the minimum possible gear up of variables, less complexity), making it useful for model selection.
All analyses were performed with SPSS for Windows, version 22.0. Significance was set at a p-value of ≤0.05.
Results
In total, 985 pregnant women in their first trimester of pregnancy were recruited. We were able to obtain the urinary iodine concentration in 970 (98.5%) women, and 945 (95.half-dozen%) also answered the standardized questionnaire.
Characteristics of the sample
The hateful age of the participants was 30.vi (4.half dozen) years, and 784 (83.0%) were Castilian. Most (696, 73.7%) were from urban areas. The breakdown past educational level was as follows: 5 (0.v%) had received no formal pedagogy, 28 (three.0%) had non completed primary school, 232 (24.half dozen%) had completed main school, 393 (41.half-dozen%) had completed secondary school, and 287 (30.4%) had a academy degree. Every bit to tobacco use, 221 (23.4%) said that they were smokers and 98 (ten.4%) ex-smokers (had stopped smoking less than ane yr previously, including those who stopped in early pregnancy); 176 (xx.4%) women continued smoking during the first trimester of pregnancy.
Urinary iodine concentration
The median overall UIC [Q1-Q3] was 172 μg/L [103.8–289.3]. UIC distribution is depicted in Fig. 1. Among the total of participants, 43.1% had a UIC <150 μg/50 and eight.8% a UIC >600 μg/L. Median UIC did not differ according to the women's identify of residence (rural/urban), place of origin, instruction level, apply of iodinated antiseptics (3.ane% of the population), or tobacco utilize (Table 1). The Spearman rho correlation coefficient between the number of cigarettes smoked and UIC level was nil (r = −0.074, p = 0.386). In total 337 (35.7%) women reported using iodized salt. The median UIC was college in this group than in nonusers (189 vs 158 μg/L; p < 0.001) (Table 2). The 442 (46.8%) women taking iodine supplementation as well showed a college median UIC than those who did non (209.five vs 140 μg/Fifty; p < 0.001 We found that an increase in milk consumption was associated with a higher UIC (139.5, 176, and 198 μg/L for 0, ane–2, and >2 spectacles/day, respectively; p = 0.003). The median UIC showed no differences in relation to fish consumption.
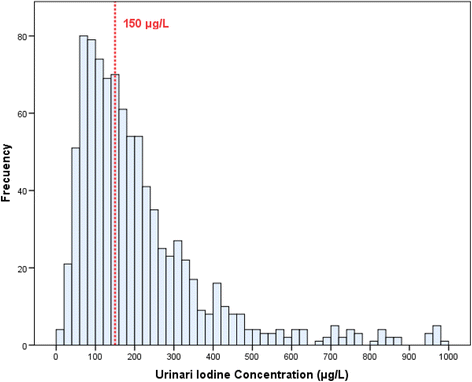
Histogram of the iodine concentration in the urine of significant women in their offset trimester of pregnancy. The discontinuous line indicates the value of urinary iodine recommended in pregnant women (≥150 mg/dL). The 43.1% of these women had UIC <150 mg/dL
Association with UIC <150 μg/L
Urinary iodine values were dichotomized into <150 μg/L and ≥150 μg/Fifty; 407 (43.1%) women had values below 150 μg/Fifty (Tables 1 and 2). On bivariate analysis, UIC <150 μg/L showed no relationship with the women's place of residence (urban or rural), identify of origin, teaching level, or smoking habit. Notwithstanding, college milk consumption (p = 0.016), iodized salt use (p < 0.001), and iodine supplementation (p < 0.001) were associated with UIC ≥150 μg/L (Table two). No associations were constitute betwixt the UIC value and fish consumption or exposure to iodinated antiseptics.
Multivariate analysis
To decide which of the variables studied were associated with an insufficient UIC, multivariate logistic regression analysis was performed using UIC <150 μg/L as the dependent variable and each of the sociodemographic variables and items on the dietary questionnaire as contained variables.
In Model 1, milk consumption had a protective effect on UIC <150 mg/50, which increased with increasing milk consumption from 1 to two to >ii glasses/day (OR 0.636 [0.45–0.xc] and 0.593 [0.37–0.95], respectively). Iodine supplementation and iodized salt intake also provided a protective effect (OR 0.410 [0.31–0.54] and OR 0.678 [0.51–0.xc], respectively) (Table 3).
In Model 2, daily consumption of i or more glasses of milk was combined with iodized salt use (Table 3). Consumption of iodized salt alone was a protective factor, but was not-significant, likely because of the relatively pocket-sized number of women who used this product (OR 0.704 [0.38–1.32]; p = 0.276). However, intake of a unmarried glass of milk yielded a protective effect (OR 0.641 [0.42–0.98]; p = 0.039), which was even greater if complemented with iodized salt consumption (OR 0.0.427 [0.27–0.66]; p < 0.001), in a fashion similar to the effect of iodine supplementation (OR 0.411 [0.31–0.54]; p < 0.001).
Discussion
The median UIC of meaning women in their starting time trimester (172 μg/Fifty) indicated an acceptable iodine nutritional status (≥150 μg/L) [14], although 43.i% of the sample showed values <150 μg/Fifty. This overall finding is consistent with the results obtained in studies conducted in adults and children (4 and 6 years sometime) from Catalonia, which reported UICs indicative of adequate iodine diet [27,28,29]. In our study, iodine condition of the meaning women included did non differ according to the place of residence (rural or urban), place of origin (native or immigrant), education level, or tobacco apply.
The median UIC nosotros found (172 μg/50) was notably higher than that obtained in a report performed in 2010 by Alvarez-Pedrerol et al. in Sabadell, an area within the setting of our study. In total, 600 women in the third trimester were included and the median UIC was 104 μg/L [23]. These differences may be explained by the lower consumption of iodized salt in that population compared to our cohort (11.5% vs 35.7%). In another study, including 267 pregnant women in the first trimester in 2 areas of Catalonia, 139 in a mount area and 128 in coastal surface area, the median UIC was found to be higher (littoral area, 142 μg/50 and mountain area 209 μg/Fifty); iodized salt was used by 36.4% and 58% of the women, respectively [24].
In a report including a sample of 1844 women in the first trimester of pregnancy from iii areas of Spain (Valencia, Guipúzcoa, Sabadell), the median UIC was lower than 150 μg/L in Valencia and Sabadell, but non in Guipúzcoa [30]. In a study conducted in Toledo (Spain) including 525 pregnant women, the median UIC was 164 μg/L [31]. The women were divided into 3 groups: 69 pregnant women who did non use iodized salt or iodine supplementation, 75 pregnant women who used iodized salt merely not supplementation, and 381 pregnant women who used potassium iodide supplementation. Median UICs in the three groups were 134.5 μg/L, 146 μg/Fifty, and 183 μg/L, respectively. In several studies, median UIC value >150 μg/L was associated with iodine supplementation [xxx,31,32,33,34].
In nearly European countries, very low median UICs have been reported, often less than 100 μg/50 [fourteen, 35], with the exception of Switzerland, Sweden, Slovenia, and some parts of Federal republic of germany [36,37,38,39,40],
In the present study, UIC assay in women who did not take potassium iodide supplements revealed that iodized salt intake forth with milk consumption was sufficient to maintain median UIC above 150 μg/L (Tabular array 3), although neither milk nor iodized table salt intake alone showed a human relationship with this value.
Multivariate analysis disclosed the contained roles of intake of milk, iodized common salt, and supplementation on the UIC. An clan was plant between milk consumption and the UIC, and when iodized common salt intake was combined with this variable, protection against iodine levels <150 μg/L was even higher, and similar to the protection obtained with supplementation (Table 3).
A similar association was detected in a recent study in northern Spain (Asturias): pregnant women who consumed 2 or more portions of dairy products per day had a UIC of 230 μg/L, whereas those who consumed less had a level of 191 μg/L. In addition, this written report constitute a median UIC ≥150 mg/50 in pregnant women who consumed iodized salt (41). In the study past Alvarez-Pedrerol et al., there was no clan between the UIC and the foods included in their survey, with the exception of milk [23]. These authors also constitute that increases in milk consumption elicited a parallel increase in mean UIC values: 0 glasses/day, 78 μg/L; 1–2 spectacles/mean solar day, 100 μg/L; and >two glasses/day, 117 μg/L. A report in pregnant women conducted in the United Kingdom also revealed a meaning positive clan between milk consumption and the UIC [41]: The mean urinary iodine/urinary creatinine ratio value was 72 μg/g when milk consumption was <140 mL/24-hour interval, which rose to 150 μg/g when consumption was more than 280 mL/day.
In Spain, the effect of milk consumption on the UIC was first detected in a study carried out in children (iv–15 years old) more than than 10 years ago [42]. Other studies including a population of adults and children who did not consume iodized common salt, reported a median UIC of more than 100 μg/L [24, 43,44,45], suggesting that other sources of iodine in add-on to iodized salt were beingness consumed, such equally dairy products. A contempo assay of 362 samples of milk from different parts of Spain showed a hateful iodine level of 259 (±58) μg/50 [46]. Furthermore, in a sample of more than 4000 Spaniards aged xviii years and older, there was a significant association between milk consumption at least once a 24-hour interval and a UIC >100 μg/L [1]. Similar results were observed in a nationwide written report conducted in a population of almost 2000 Spanish children [47]. The role of milk as a source of iodine, as was seen in Spain, is recognized in other European countries [48,49,50,51,52,53]. All the same, the "accidental iodination" of milk, such every bit that occurring in the Uk [48], implies a risk of considerable variations in iodine content if there is no regulation and command. Australia has seen a drop in iodine diet due to a decreased iodine content in its milk supply [54, 55].
Iodized table salt is the food traditionally recommended by the WHO and ICCIDD to ensure a proper level of iodine nutrition in the population [56]. The risk of iodine deficiency could be resolved if at least 90% of households consumed iodized salt. In Spain, still, iodized salt consumption in households is less than 50% and in the case of pregnant women, 35.5% [1]. Every bit in other studies performed in our geographic area [23, 24], no clan was found between fish consumption and the UIC in the pregnant women studied.
About nine% of our population had farthermost UIC values (>600 μg/L). Although potassium iodide supplementation was higher in this group, it is difficult to aspect this to such a high UIC. Yet, seaweed consumption, awarding of iodized antiseptics in pregnancy [57], and utilise of salt with excessive iodination could justify UIC values >600 mg/dL.
Some studies take reported an association between smoking and an increased adventure of goiter or other thyroid problems [18, 20, 24, 51, 58], but nosotros did not detect a pregnant effect of tobacco use on UIC values.
Determination
In conclusion, the overall median UIC of women in their kickoff trimester of pregnancy found in our study is indicative of acceptable iodine nutritional status according to the criteria established past the WHO and ICCIDD, although 43.1% were found to have suboptimal levels. Simultaneous consumption of iodized salt and milk was equally effective every bit potassium iodide supplementation to accomplish acceptable iodine diet. The iodine concentration in salt in Kingdom of spain (60 ppm) and the high concentration of iodine in cow milk, which has increased in recent years, may explicate these findings.
Women of childbearing age in our region should consume iodized common salt and milk to maintain an optimal UIC, particularly those who are planning a pregnancy. A good intra-thyroidal deposit of iodine enables the gland to adapt and respond better to the physiologic changes that occur during pregnancy. If women utilize these iodine-rich products in the twelvemonth before and during pregnancy, their iodine status will likely be acceptable and iodine supplementation will not exist necessary. Public wellness campaigns should be developed in our setting to promote consumption of iodized salt. Moreover, our national food agencies should ensure adequately iodized common salt and regular monitoring of iodine content in milk.
References
-
Soriguer F, García-Fuentes E, Gutierrez-Repiso C, Rojo-Martínez K, Velasco I, Goday A, Bosch-Comas A, Bordiú Due east, Calle A, Carmena R, Casamitjana R, Castaño L, Castell C, Catalá 1000, Delgado East, Franch J, Gaztambide S, Girbés J, Gomis R, Gutiérrez G, López-Alba A, Martínez-Larrad MT, Menéndez E, Mora-Peces I, Ortega E, Pascual-Manich G, Serrano-Rios M, Valdés S, Vázquez JA, Vendrell J. Iodine intake in the adult population. Di@bet.Es written report. Clin Nutr. 2012;31:882–8.
-
Morreale de Escobar G, Escobar del Rey F. Metabolismo de las hormonas tiroideas y el yodo en el embarazo. Razones experimentales para mantener una ingesta de yodo adecuada en la gestación. Endocrinol y Nutr. 2008;55(Supple):7–17.
-
Glinoer D. The regulation of thyroid office during normal pregnancy: importance of the iodine diet status. Best Pract Res Clin Endocrinol Metab. 2004;18:133–52.
-
Glinoer D. The importance of iodine nutrition during pregnancy. Public Heal Nutr. 2007;x:1542–6.
-
Donnay Candil S. Uso racional del yoduro potásico durante el embarazo y la lactancia. Endocrinol y Nutr. 2008;55(Supple):29–34.
-
Delange F. Optimal iodine nutrition during pregnancy, lactation and the neonatal period. Int J Endocrinol Metab. 2004;2:1–12.
-
Morreale de Escobar Thou, Obregon M, Escobar del Rey F. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–48.
-
Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early encephalon evolution. Eur J Endocrinol. 2004;151(Suppl):U25–37.
-
Zimmermann MB. The role of iodine in human growth and development. Semin Cell Dev Biol. 2011;22:645–52.
-
Velasco I. Anomalías prenatales asociadas a la deficiencia de yodo. Progr Diag Trat Prenat. 2005;17:123–8.
-
Vermiglio F, Lo Presti VP, Moleti M, Sidoti M, Tortorella K, Scaffidi K, Castagna MG, Mattina F, Violi MA, Crisà A, Artemisia A, Trimarchi F. Attending arrears and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–60.
-
Berbel P, Mestre JL, Santamaria A, Palazon I, Franco A, Graells Yard, Gonzalez-Torga A, de Escobar GM. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the commencement month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19:511–ix.
-
Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck K-SSMPF, Hofman A, Jaddoe VVW, Visser Westward, Steegers EAP, Verhulst FC, de Rijke YB, Tiemeier H. Maternal thyroid part during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. 2010;95:4227–34.
-
ICCIDD: Iodine requeriments in pregnancy and infancy. 2007;:1–2. https://www.thyroid.org/wp-content/uploads/professionals/educational activity/IDD_NL_Feb07.pdf. Accessed 22 July 2017.
-
Laurberg P, Andersen Due south, Bjarnadottir RI, Carle A, Hreidarsson A, Knudsen Northward, Ovesen L, Pedersen I, Rasmussen L. Evaluating iodine deficiency in pregnant women and young infants-complex physiology with a risk of misinterpretation. Public Heal Nutr. 2007;ten:1547–52.
-
Puig-Domingo Chiliad, Vila L. Iodine status, thyroid and pregnancy. Hot Thyroidol. 2010; (5/10). http://world wide web.ibrarian.net/navon/paper/Iodine_status__thyroid_and_pregnancy.pdf?paperid=19279198. Accessed 22 July 2017.
-
Bertelsen JB, Hegedüs 50. Cigarette smoking and the thyroid. Thyroid. 1994;4:327–31.
-
Männistö T, Hartikainen AL, Vääräsmäki Grand, Bloigu A, Surcel HM, Pouta A, Järvelin MR, Ruokonen A, Suvanto E. Smoking and early pregnancy thyroid hormone and anti-thyroid antibiotic levels in euthyroid mothers of the Northern Finland Birth Cohort 1986. Thyroid. 2012;22(9):944–50.
-
McDonald SD, Walker MC, Ohlsson A, Spud KE, Beyene J, Perkins SL. The effect of tobacco exposure on maternal and fetal thyroid part. Eur J Obstet Gynecol Reprod Biol. 2008;140:38–42.
-
Shields B, Hill A, Bilous M, Knight B, Hattersley AT, Bilous RW, Vaidya B. Cigarette smoking during pregnancy is associated with alterations in maternal and fetal thyroid office. J Clin Endocrinol Metab. 2009;94:570–4.
-
Vanderver GB, Engel A, Lamm S. Cigarette smoking and iodine equally hypothyroxinemic stressors in U.S. women of childbearing age: a NHANES 3 assay. Thyroid. 2007;17:741–six.
-
Laurberg P, Nøhr SB, Pedersen KM, Fuglsang E. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab. 2004;89:181–vii.
-
Alvarez-Pedrerol M, Ribas-Fito Due north, Garcia-Esteban R, Rodriguez A, Soriano D, Guxens Grand, Mendez One thousand, Sunyer J. Iodine sources and iodine levels in significant women from an surface area without known iodine deficiency. Clin Endocrinol. 2010;72:81–6.
-
Vila 50, Serra-Prat M, de Castro A, Palomera Eastward, Casamitjana R, Legaz Thou, Barrionuevo C, Munoz JA, Garcia AJ, Lal-Trehan S, Garcia A, Duran J, Puig-Domingo G. Iodine nutritional status in pregnant women of two historically unlike iodine-deficient areas of Catalonia, Kingdom of spain. Nutrition. 2011;27:1029–33.
-
Prieto G, Torres MT, Francés L, Falguera G, Vila L, Manresa JM, Casamitjana R, Barrada JR, Acera A, Guix D, Torrent A, Grau J, Torán P. Nutritional status of iodine in pregnant women in Catalonia (Spain): study on hygiene-dietetic habits and iodine in urine. BMC Pregnancy Childbirth. 2011;11:17.
-
Pino South, Fang SL, Braverman LE. Ammonium persulfate: a new and safe method for measuring urinary iodine by ammonium persulfate oxidation. Exp Clin Endocrinol Diabetes. 1998;106(Suppl):S22–vii.
-
Serra-Prat M, Diaz E, Verde Y, Gost J, Serra E, P-D Thousand. Prevalence of iodine deficiency and related factors in four year-erstwhile schoolchildren. Med Clin. 2003;120:246–9.
-
Vila Fifty, Castell C, Wengrovicz S, de L North, Casamitjana R. Estudio de la yoduria de la poblacion catalana adulta. Med Clin. 2006;127:730–3.
-
Capdevila Bert R, Marsal Mora JR, Pujol Salud J, Anguera Farran R. Prevalence study of iodine deficiency in a 6-year-old school population. An Pediatr. 2010;72:331–8.
-
Rebagliato Thousand, Murcia Chiliad, Espada K, Alvarez-Pedrerol M, Bolumar F, Vioque J, Basterrechea K, Blarduni Due east, Ramon R, Guxens One thousand, Foradada CM, Ballester F, Ibarluzea J, Sunyer J. Iodine intake and maternal thyroid function during pregnancy. Epidemiology. 2010;21:62–9.
-
Marco A, Vicente A, Castro Eastward, Eva PC, Rodriguez O, Merchan MA, Sastre J, Canovas B, Maqueda E, Pena V, Lopez J. Patterns of iodine intake and urinary iodine concentrations during pregnancy and claret thyroid-stimulating hormone concentrations in the newborn progeny. Thyroid. 2010;20:1295–9.
-
Santiago P, Berrio M, Olmedo P, Velasco I, Sanchez B, Garcia E, Martinez J, Soriguer F. Reference values for thyroid hormones in the population of meaning women in Jaen (Spain). Endocrinol Nutr. 2011;58:62–7.
-
Jaen Diaz JI, Lopez de Casto F, Cordero Garcia B, Santillana Balduz F, Martin Dal Gesso C. Thyroid disorders and iodine nutritional status in the first trimester of pregnancy. Endocrinol Nutr. 2008;55:196–201.
-
Santiago P, Velasco I, Muela JA, Sánchez B, Martínez J, Rodriguez A, Berrio M, Gutierrez-Repiso C, Carreira Chiliad, Moreno A, García-Fuentes Eastward, Soriguer F. Infant neurocognitive development is independent of the use of iodised salt or iodine supplements given during pregnancy. Br J Nutr. 2013;110(5):831–9.
-
Zimmermann Chiliad, Delange F. Iodine supplementation of pregnant women in Europe: a review and recommendations. Eur J Clin Nutr. 2004;58:979–84.
-
Zimmermann MB, Aeberli I, Torresani T, Burgi H. Increasing the iodine concentration in the Swiss iodized table salt program markedly improved iodine condition in pregnant women and children: a 5-y prospective national report. Am J Clin Nutr. 2005;82:388–92.
-
Andersson M, Aeberli I, Wust N, Piacenza AM, Bucher T, Henschen I, Haldimann Thou, Zimmermann MB. The Swiss iodized salt program provides acceptable iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods every bit well equally their mothers are iodine deficient. J Clin Endocrinol Metab. 2010;95:5217–24.
-
Elnagar B, Eltom A, Broad Fifty, Gebre-Medhin Chiliad, Karlsson FA. Iodine condition, thyroid function and pregnancy: study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–v.
-
Fister P, Gaberscek Due south, Zaletel K, Krhin B, Gersak K, Hojker Due south. Thyroid book changes during pregnancy and after delivery in an iodine-sufficient Republic of Slovenia. Eur J Obs Gynecol Reprod Biol. 2009;145:45–eight.
-
Buhling KJ, Schaff J, Bertram H, Hansen R, Muller C, Wascher C, Heinze T, Dudenhausen JW. Supply of iodine during pregnancy--an inventory in Berlin, Deutschland. Z Geburtshilfe Neonatol. 2003;207:12–6.
-
Bath SC, Furmidge-Owen VL, Redman CW, Rayman MP. Gestational changes in iodine condition in a cohort report of pregnant women from the United kingdom of great britain and northern ireland: flavour as an effect modifier. Am J Clin Nutr. 2015;101:1180–7.
-
Millon JM, Soriguer F, Munoz R, Mancha I, Gomez-Huelga R, Goiburu Eastward, Garcia-Almeida JM, Gonzalez-Romero S, Rojo-Martinez G. Determinant factors of ioduria in a scholar population in the south of Spain. Endocrinol y Nutr. 2001;48:104–ix.
-
Vila Ballester LL, Subirats Bayego Due east, Vila Subirana T, Margalef Mir North, Vallescar R, de Leiva A. An endemic goiter report of a population in the Pyrenees (Cerdanya-Girona). An Med Interna. 1999;16:338–44.
-
Peris RB, Atienzar HN, Merchante Alfaro AA, Calvo RF, Tenias Burillo JM, Selfa MS, Lopez Garcia MJ. Bocio endemico y deficit de yodo: ¿sigue siendo una realidad en España? An Pediatr. 2006;65:234–twoscore.
-
Delgado E, D-C FJ, Tarton T, ML B, MM V. Erradicacion de los trastornos por deficiencia de yodo en Asturias (España): 18 años de yodoprofilaxis con sal. Endocrinol y Nutr. 2004;51:492–6.
-
Soriguer F, Gutierrez-Repiso C, Gonzalez-Romero S, Olveira G, Garriga MJ, Velasco I, Santiago P, de Escobar GM, Garcia-Fuentes E. Iodine concentration in cow's milk and its relation with urinary iodine concentrations in the population. Clin Nutr. 2011;thirty:44–8.
-
Vila L, Donnay Southward, Arena J, Arrizabalaga JJ, Pineda J, Garcia-Fuentes E, García-Rey C, Marín JL, Serra-Prat Thousand, Velasco I, López-Guzmán A, Luengo LM, Villar A, Muñoz Z, Bandrés O, Guerrero E, Muñoz JA, Moll One thousand, Vich F, Menéndez E, Riestra M, Torres Y, Beato-Víbora P, Aguirre M, Santiago P, Aranda J, Gutiérrez-Repiso C. Iodine status and thyroid function among Castilian schoolchildren aged 6-7 years: the Tirokid study. Br J Nutr. 2016;115:1623–31.
-
Phillips DI. Iodine, milk, and the emptying of owned goitre in United kingdom: the story of an accidental public health triumph. J Epidemiol Community Heal. 1997;51:391–3.
-
Girelli ME, Money P, Mian C, Nacamulli D, Zambonin L, Piccolo Thousand, Vianello-Dri A, Gottardo F, Busnardo B. Milk represents an of import source of iodine in schoolchildren of the Veneto region, Italy. J Endocrinol Investig. 2004;27:709–13.
-
Lamberg BA. Endemic goitre in Finland and changes during 30 years of iodine prophylaxis. Endocrinol Exp. 1986;20:35–47.
-
Rasmussen LB, Ovesen Fifty, Bulow I, Jorgensen T, Knudsen Due north, Laurberg P, Pertild H. Dietary iodine intake and urinary iodine excretion in a Danish population: outcome of geography, supplements and food choice. Br J Nutr. 2002;87:61–9.
-
Zamrazil V, Bilek R, Cerovska J, Delange F. The elimination of iodine deficiency in the Czech Republic: the steps toward success. Thyroid. 2004;fourteen:49–56.
-
Dahl Fifty, Opsahl JA, Meltzer HM, Julshamn One thousand. Iodine concentration in Norwegian milk and dairy products. Br J Nutr. 2003;90:679–85.
-
Li M, Waite KV, Ma Grand, Eastman CJ. Declining iodine content of milk and re-emergence of iodine deficiency in Australia. Med J Aust. 2006;184:307.
-
Li M, Eastman CJ, Waite KV, Ma G, Zacharin MR, Topliss DJ, Harding PE, Walsh JP, Ward LC, Mortimer RH, Mackenzie EJ, Byth K, Doyle Z. Are Australian children iodine scarce? Results of the Australian National Iodine Diet Report. Med J Aust. 2006;184:165–9.
-
WHO, ICCIDD, UNICEF: Cess of iodine deficiency disorders and monitoring their elimination. A guide for programme managers. http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf. 2007 (Thirst). Accessed 22 July 2017.
-
Velasco I, Naranjo S, Lopez-Pedrera C, Garriga MJ, Garcia-Fuentes East, Soriguer F. Employ of povidone-iodine during the first trimester of pregnancy: a right practice? BJOG. 2009;116:452–five.
-
Chanoine JP, Toppet Five, Bourdoux P, Spehl M, Delange F. Smoking during pregnancy: a significant crusade of neonatal thyroid enlargement. Br J Obs Gynaecol. 1991;98:65–8.
Acknowledgements
We wish to thank the pregnant women for participating as volunteers, and the midwives who collaborated in the data collection.
We appreciate the support provided by collaborators from The IODEGEST Study Grouping.
The IODEGEST Study Grouping
Montse Abella, Nuria Sampedro, Glòria Miralpeix, Montse Villanueva, Concepción Manzano, Judit Cos (PASSIR Sabadell); Pilar Soteras, Fermina Casas, Coloma Graells, Mireia Llucià, Rosalia Ibars (PASSIR Cerdanyola); Encarna López, Montserrat Manzanares, Irene Lorente, Eva Artieda, Meritxell Casajoana, Dolors Muñoz, Llucia Burgos, Angelica Hidalgo (PASSIR Mollet); Anna Fusté, Dolors Lladó, Rosa Subirats, Angelina Masoliver, Imma Trujillo, Rosa Banús, Dolors Salas, Montse Pujol, Dolors Grau, Roser Sanglas, Anabel Mayos (PASSIR Osona); Náyade Crespo, Rosa Codina, Rosa Forn, Montserrat Galí, Antonia Hidalgo, Teresa Macià, Mercè Vendrell, Montserrat Sallent, Montserrat Ribera, Rosa Oller, Teresa Riba, Esther Romero, Adelaida Expósito, Encarnació Santaeulàlia, Anna Vilaseca (PASSIR Bages); Dolors Guix, Gemma Olivera, Merche García, Rosa Sans, Marta Roman, Mª Jose Vila, Maite Martinez, Esther Serrano, Sonia Díaz, Carolina Alcaine, Susana Sancho, Remei Fenollosa, Encarna Gascón, Núria Risques, Araceli Santamaria, Remei Corominas, Xavi Espada, Maria Helena Perez, Concepción de la Fuente, Assumpta Prats, Maria Rosa Cabedo, Carme Magem, Mercedes Vigil, Carmen Biern, Montse Bach, Joana Relat. (PASSIR Granollers); Engracia Coll, Carmen Bayascas, Olga Ezquerro, Patricia Reategui, Anna Campos, Rosa Bach (PASSIR Mútua Terrassa); Inés Molina, Eva Martinez, Anna Bartolí, Rosa Ferrer, Rocio Hernandez. (PASSIR Anoia).
Funding
This project was awarded a grant from the Castilian Ministry of Health, from the Carlos Three Health Plant (PI 07/1265) and has been registered at the Trial Registration: Clinical Trials.gov (Identifier NCT01301768).
Availability of information and materials
Fastened dataset as Boosted file one.
Author data
Affiliations
Consortia
Contributions
MTT have made substantial contributions to the formulation and design, acquisition of data, assay and interpretation of data. GF, GP have made substantial contributions to the conception and design. LF, LLV take made substantial contributions to the formulation and blueprint, and estimation of information. All authors read and canonical the concluding manuscript.
Respective author
Ethics declarations
Authors' information
Not applicative.
Ideals approval and consent to participate
All women consent to participate. The study was approved by the Clinical Research Ethics Committee at the Chief Care Research Institute (IDIAP) Jordi Gol.
Consent for publication
Non applicable.
Competing interests
The authors declare that they accept no conflicts of interest.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Rights and permissions
Open up Access This article is distributed nether the terms of the Artistic Commons Attribution iv.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted utilise, distribution, and reproduction in any medium, provided yous give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Artistic Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/naught/1.0/) applies to the data fabricated bachelor in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Torres, M.T., Francés, L., Vila, L. et al. Iodine nutritional status of women in their offset trimester of pregnancy in Catalonia. BMC Pregnancy Childbirth 17, 249 (2017). https://doi.org/10.1186/s12884-017-1423-4
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12884-017-1423-4
Keywords
- Iodine Nutrition Condition
- International Council For Command Of Iodine Deficiency Disorders (ICCIDD)
- Iodized Common salt
- Urinary Iodine Concentration
- Iodine Supplementation
Source: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-017-1423-4
0 Response to "what recommendation is made for pregnant women to ensure they consume adequate iodine?"
Enregistrer un commentaire